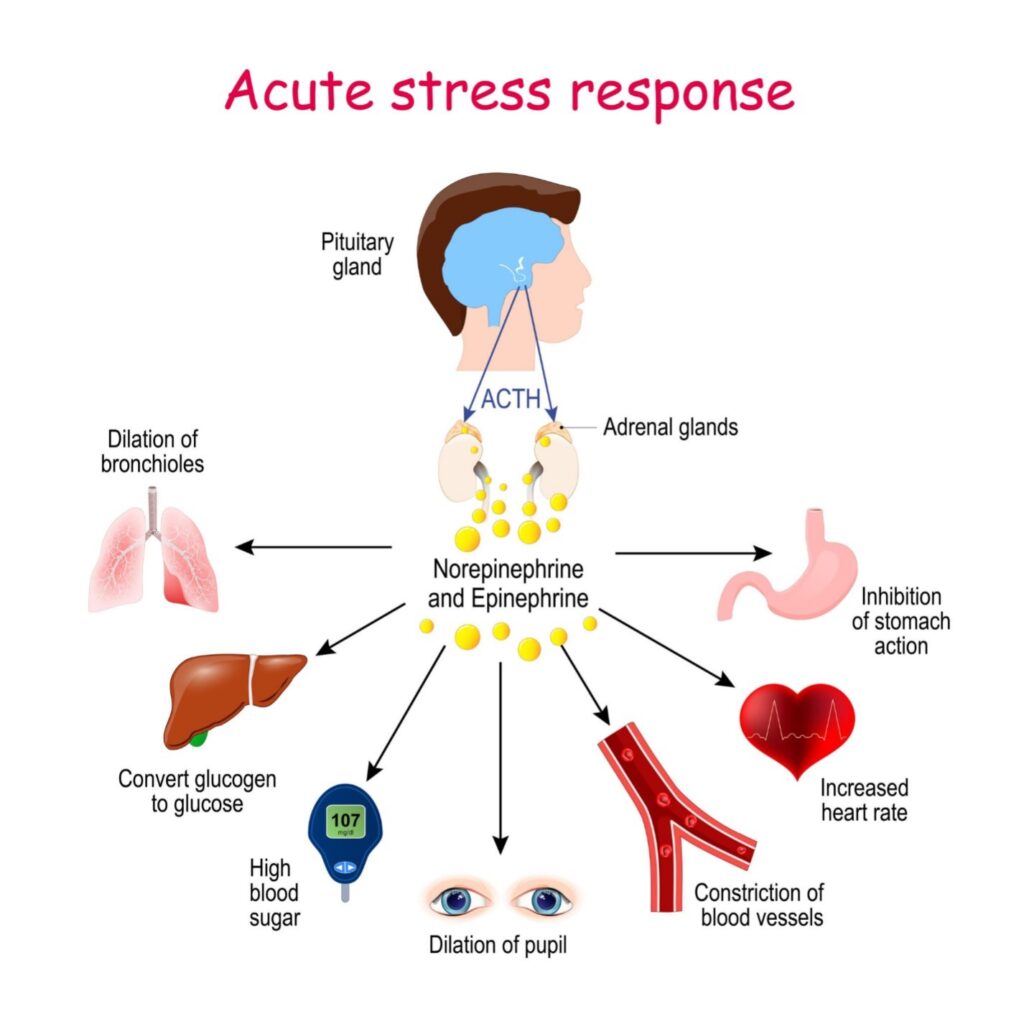
The immune system reacts differently depending on whether the stress is a sudden “fight-or-flight” moment or a lingering burden.
The Mechanism: How Stress Suppresses Immunity
The primary driver of stress-induced immunosuppression is the HPA Axis (Hypothalamic-Pituitary-Adrenal Axis) and its end-product: Cortisol.
The Chain Reaction
- The Trigger: When you are stressed, your brain (hypothalamus) signals your pituitary gland, which then signals your adrenal glands.
- The Release: Your adrenal glands release glucocorticoids (mainly cortisol in humans).
- The Suppression: Cortisol is useful in small doses because it lowers inflammation (which is why we put hydrocortisone cream on bug bites). However, biologically, cortisol effectively tells your immune system to “stand down” to save energy for the physical exertion of running away or fighting.
Cellular Impact
When cortisol floods your system for weeks or months, it causes specific damage to immune cells:
- T-Cell Suppression: Cortisol suppresses the production of Interleukin-2 (IL-2), a molecule T-cells need to multiply. Without IL-2, your “infantry” of T-cells cannot expand to fight an invader.
- Apoptosis (Cell Death): High concentrations of cortisol can literally cause immature T-cells and B-cells to self-destruct.
- Involution of the Thymus: Chronic stress can physically shrink the thymus gland, the organ where T-cells mature.
- The “Stress Paradox”: Why am I inflamed if I am suppressed?
You might wonder: If stress suppresses the immune system (which usually lowers inflammation), why is stress linked to inflammatory diseases like heart disease and autoimmune flare-ups?
This occurs due to Glucocorticoid Resistance.
- Phase 1: Stress raises cortisol -> Immune system is suppressed.
- Phase 2 (Burnout): If cortisol stays high for too long, immune cells eventually stop listening to the signal. They “downregulate” their cortisol receptors.
- Result: The “off switch” for inflammation is broken. Your immune system is now suppressed regarding fighting viruses (low antiviral defense) but hyperactive regarding creating inflammation (high cytokine levels). This is the worst of both worlds.
Summary of Health Consequences
-
- Latent Virus Reactivation: Viruses that stay dormant in your body (like Epstein-Barr, Herpes Simplex, or Varicella) often reactivate during exams, divorce, or intense periods of work because the specific T-cells holding them back are suppressed.
- Vaccine Failure: Studies have shown that chronically stressed individuals (such as long-term caregivers) produce fewer antibodies in response to flu shots compared to non-stressed controls.
- Slower Wound Healing: High cortisol slows the production of cytokines needed to repair tissue.
Do People with Stress or Depression Tolerate Chemotherapy Worse?
Recent research indicates that people with high levels of stress or depression tolerate chemotherapy worse than those without.
This is not just “all in your head.” There are concrete biological reasons why your body physically struggles to process chemotherapy when you are under chronic stress. It affects everything from how fast your liver breaks down the drugs to how severely you feel the side effects.
Here is the breakdown of why stress makes chemotherapy harder on the body.
- The Metabolic Impact (How your body processes the drug)
This is the most critical and often overlooked factor. Your liver uses specific enzymes (primarily the Cytochrome P450 family) to metabolize chemotherapy drugs.
- The Mechanism: Stress hormones like cortisol interact with these liver enzymes (specifically CYP3A4).
- The Consequence: This can make your metabolism “erratic.”
- If metabolism speeds up: Your body clears the drug too fast, potentially making the treatment less effective.
- If metabolism slows down: The drug stays in your system longer than intended, leading to higher toxicity. This causes more severe side effects like nerve damage (neuropathy), intense nausea, and plummeting white blood cell counts.
- The “Double-Hit” to the Immune System
As we discussed, chronic stress suppresses the immune system. Chemotherapy also suppresses the immune system (specifically by killing fast-dividing cells like white blood cells).
- Infection Risk: A stressed patient essentially suffers a “double-hit.” The chemotherapy lowers their defenses, and their own stress hormones (cortisol) prevent the remaining immune cells from rallying effectively.
- Recovery Time: This often leads to longer recovery times between chemo cycles, forcing doctors to delay treatments or lower the dose, which can impact the overall success of the cancer therapy.
- Symptom Amplification (The “Chemo Brain” Overlap)
Depression and chemotherapy side effects often look identical, creating a feedback loop where one worsens the other.
- Fatigue & Fog: Both chemotherapy and depression cause “brain fog” (cognitive impairment). When combined, patients report significantly higher rates of memory loss and inability to concentrate.
- Pain Perception: Depression alters the brain’s neurotransmitters (like serotonin and norepinephrine) that regulate pain. A depressed nervous system is physically more sensitive to pain, making side effects like joint pain or neuropathy feel more intense than they would in a non-depressed patient.
- Drug Resistance
Perhaps most concerning is that stress hormones can protect the cancer cells themselves.
- Cellular Armor: Studies suggest that stress hormones (catecholamines like adrenaline) can trigger signaling pathways inside cancer cells that make them more resistant to cell death (apoptosis).
- DNA Repair: Stress hormones can interfere with DNA repair mechanisms. Since many chemo drugs work by damaging cancer DNA, stress can inadvertently help cancer cells survive the attack.
Important Note on Steroids
You might notice that doctors often give you steroids (like Dexamethasone) along with chemotherapy.
- The Difference: These are controlled, short-term doses designed to stop inflammation and nausea.
- The Problem: Chronic stress produces unregulated, long-term cortisol that causes the damage described above.